For healthcare professionals only
You are viewing the Novo Nordisk Virtual platform, provided to non-US health care professionals from around the world. By accessing this site and materials you accept this legal notice and expressly confirm your status as a healthcare professional.
This site is not country-specific and therefore may contain information which is not applicable to your country. Therefore, before prescribing any product, always refer to local materials such as the prescribing information and/or the Summary of Product Characteristics.
This site is not intended to provide medical advice and/or treatment guidance. Novo Nordisk accepts no liability for the accuracy, completeness or use of the information, and disclaims any liability to update the information contained on this site.
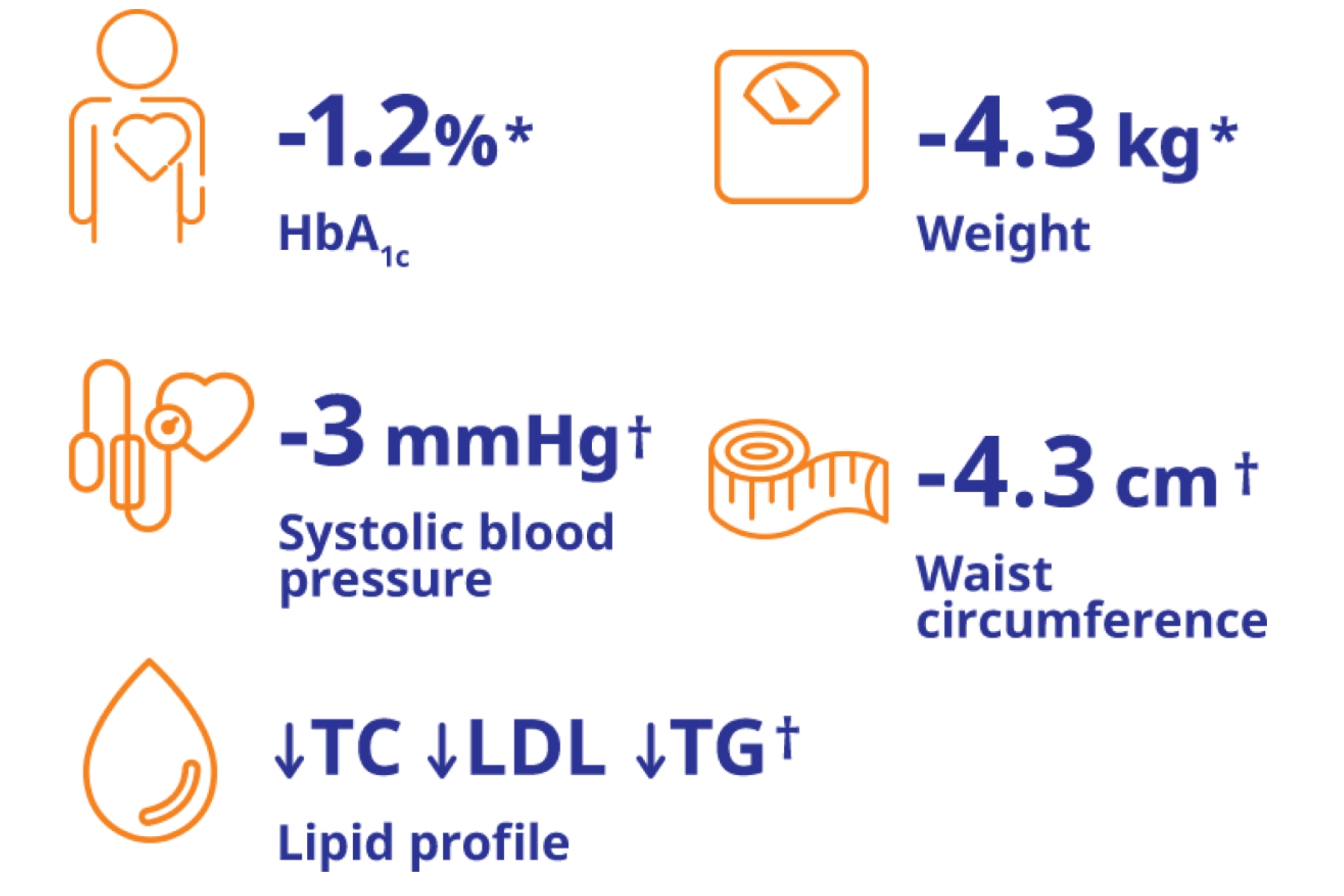
*Weight reduction results are from PIONEER 4, a 52-week, double-blind, double-dummy trial in 711 adult patients with type 2 diabetes that compared the efficacy and safety of RYBELSUS® vs liraglutide and placebo.4
GLP-1 RA: glucagon-like peptide-1 receptor agonist.
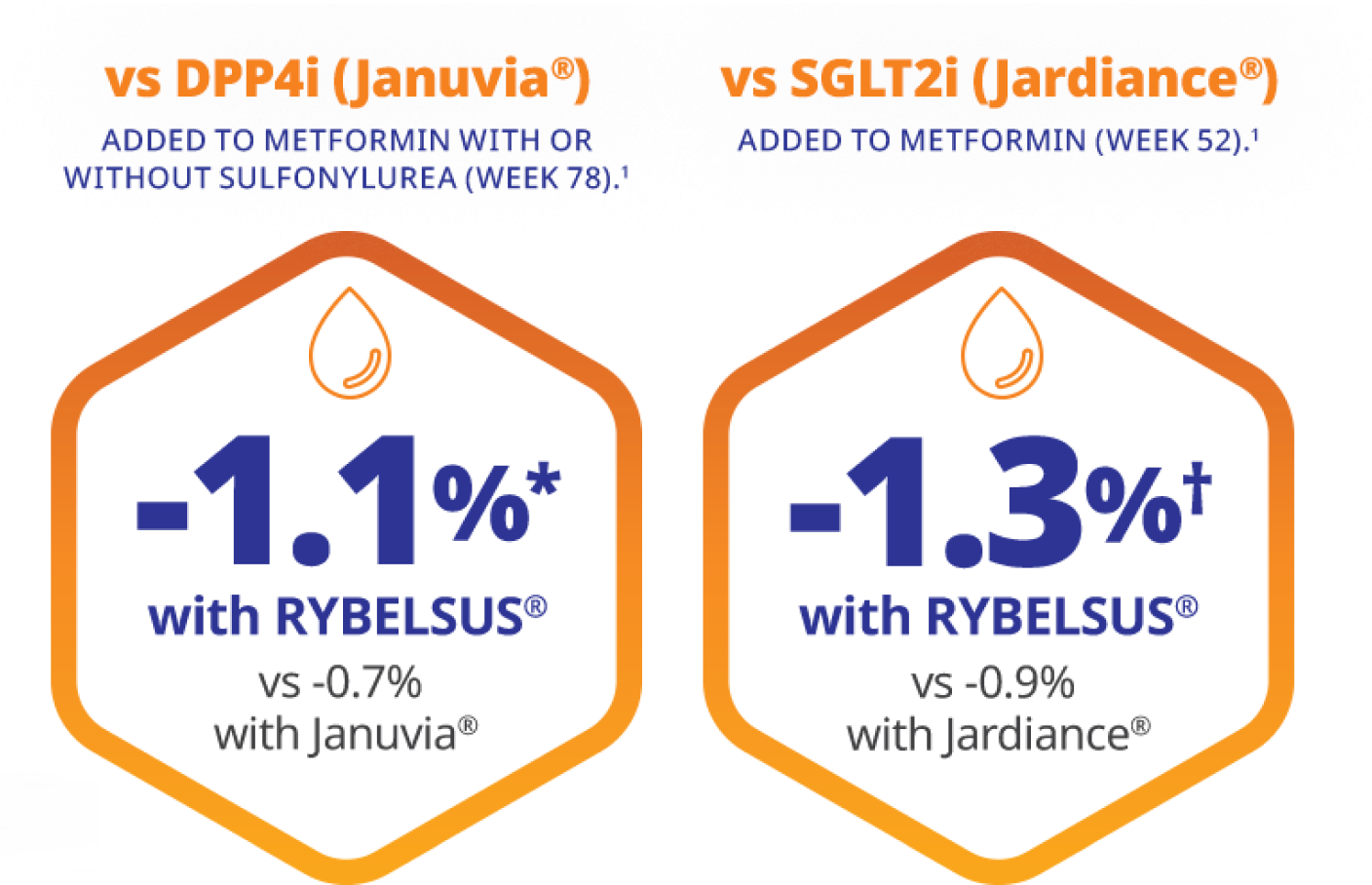
*Results are from a 78-week, randomised, double-blind, active-controlled trial in 1864 adult patients with type 2 diabetes.1,3
Patients achieved -1.1% HbA1c reduction with RYBELSUS® 14 mg (Baseline: 8.3%, n=465, *p<0.05) vs -0.7% with Januvia® 100 mg (Baseline: 8.3%, n=467).1,2
†Results are from a 52-week, randomised, open-label, active-controlled trial in 822 adult patients with type 2 diabetes.1,4
Patients achieved -1.3 HbA1c reduction with RYBELSUS® 14 mg (Baseline: 8.1%, n=411, *p<0.05) vs -0.9% with Jardiance® 25 mg (Baseline: 8.1%, n=410).1,3
Model is not a real patient and situations shown do not reflect model's experience.
DPP4i: dipeptidyl peptidase-4 inhibitor; SGLT2i: sodium-glucose cotransporter 2 inhibitor.
*(Baseline 92.2 kg, n=285) *p<0.05 vs -3.0 kg with Victoza® 1.8 mg (baseline 95.5 kg, n=284) vs -1.0 kg with placebo (Baseline 93.2 kg, n=142). Results are from PIONEER 4, a 52-week, double-blind, double-dummy trial in 711 patients with type 2 diabetes.4
Model is not a real patient and situations shown do not reflect model's experience
*p<0.05.
†Changes are significant vs liraglutide and placebo.
Results are from PIONEER 4, a 52-week, double-dummy trial in 711 adult patients with type 2 diabetes that compared the efficacy and safety of RYBELSUS® vs liraglutide and placebo. Down arrow indicates significantly greater decrease.4
LDL: low density lipoprotein; TC: total control; TG: triglycerides.
RYBELSUS® [summary of product characteristics]. Bagsværd, Denmark: Novo Nordisk A/S; March 2024.
Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019 Apr 16;321(15):1466-1480.
Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019 Dec;42(12):2272-2281.
Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394(10192):39-50.
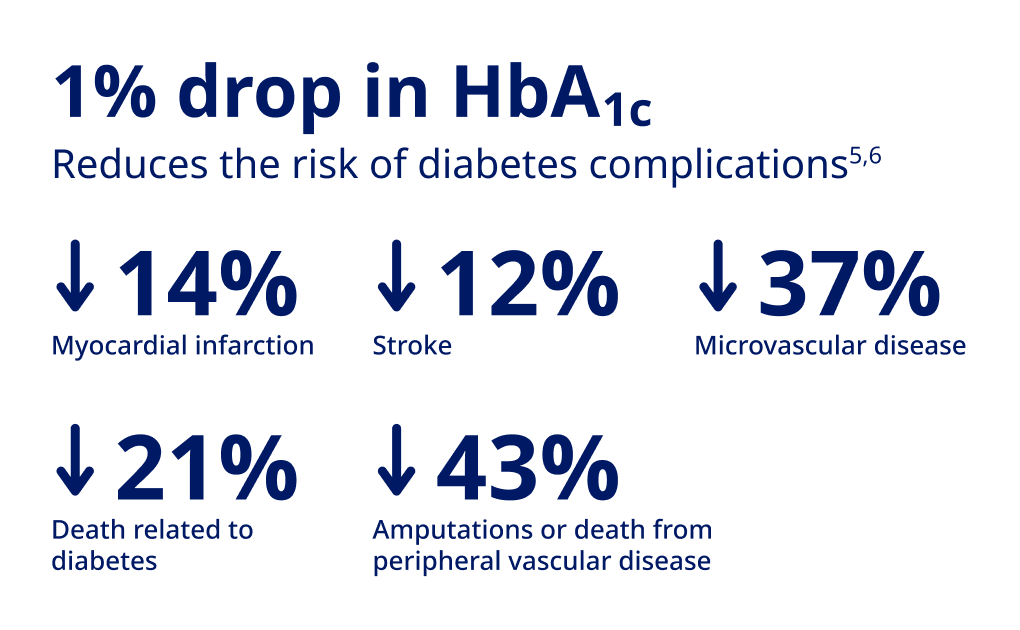
Lind M, Imberg H, Coleman RL, Nerman O, Holman RR. Historical HbA1c values may explain the type 2 diabetes legacy effect: UKPDS 88. Diabetes Care. 2021;44:1-7.
Stratton IM. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective Observational Study. BM}. 2000;321(7258):405-412.
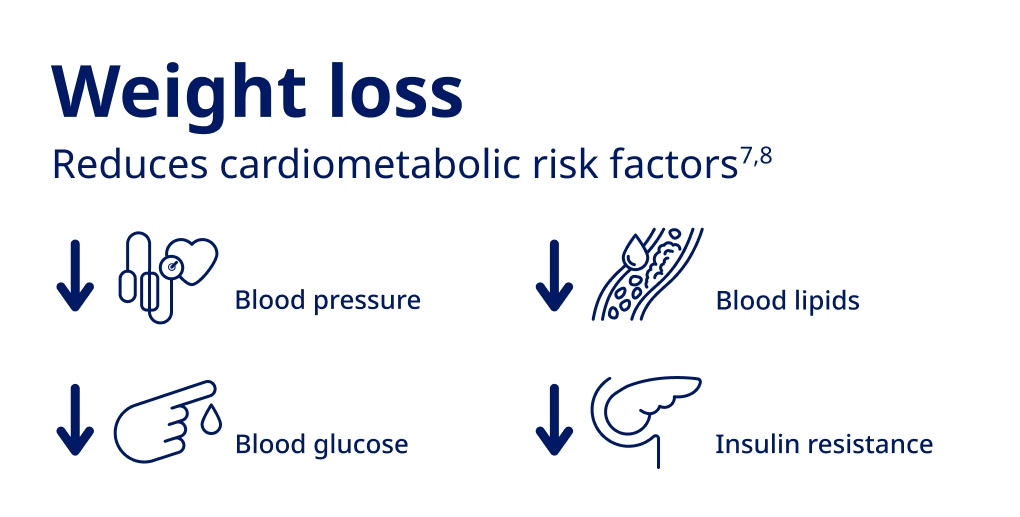
Burke GL, Bertoni AG, Shea S, et al. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease. Archives of Internal Medicine. 2008;168(9):928.
Mendis S, Puska P, Norrving B. Global Atlas on Cardiovascular Disease Prevention and Control. World Health Organization, Geneva 2011.
Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectrum. 2017;30(3):202-210.
Cornell S. A review of GLP-1 receptor agonists in type 2 diabetes: a focus on the mechanism of action of once-weekly agents.Journal of Clinical Pharmacy and Therapeutics. 2020;45(51):17-27.
Rasalam R, Barlow J, Kennedy M, et al. GLP-1 Receptor Agonists for Type 2 Diabetes and Their Role in Primary Care: An Australian Perspective. Diabetes Ther. 2019;10(4):1205–1217.
American Diabetes Association. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. Clin Diabetes. 2020;38:10-38.
Davies MJ, D'Alessio DA, Fradkin J et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669-2701.
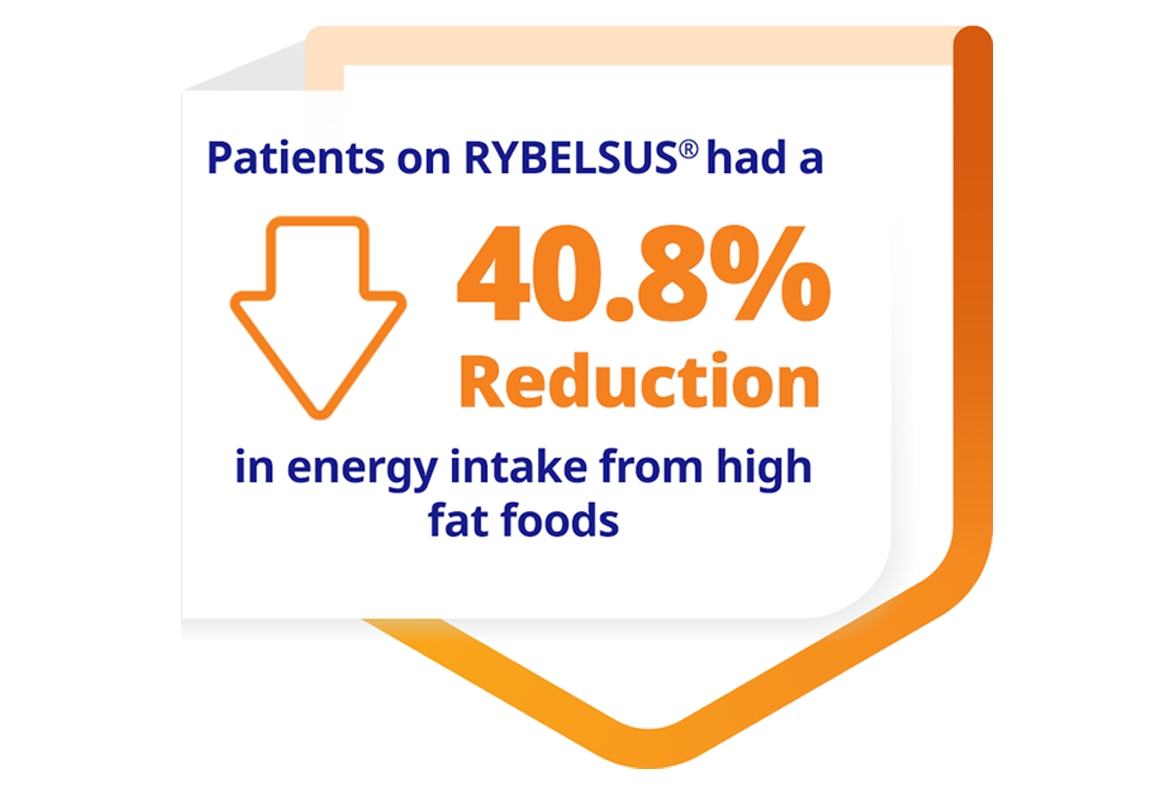
Gibbons C, Blundell J, Tetens Hoff S, Dahl K, Bauer R, Bækdal T.
Effects of oral semaglutide on energy intake, food preference, appetite, control of eating and body weight in subjects with type 2 diabetes. Diabetes, Obesity and Metabolism. 2020;23(2):581-588.
Data on file. Novo Nordisk A/S Bagsværd, Denmark. 2016-2021.
Buckley ST, Bækdal TA, Vegge A, et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018 Nov 14;10(467):eaar7047.
FOR HEALTHCARE PROFESSIONALS ONLY
The information contained in this site is intended for healthcare professionals only outside of the United States of America. This site is not intended to provide medical advice and/or treatment guidance. Only a physician can determine whether a specific product is correct for a particular patient. This site is not country-specific and therefore may contain information which is not applicable to your country. Novo Nordisk accepts no liability for the accuracy, completeness or use of this information, and disclaims any liability to update the information contained on this site. By accessing this site and materials you accept this legal notice and expressly confirm your status as a healthcare professional. Any images shown are models and not real patients.
The Summary of Product Characteristics (SmPC) is based on the EU SmPC as of March 2024. Registration conditions differ internationally. Always refer to the full local SmPC before prescribing.