For healthcare professionals only
You are viewing the Novo Nordisk Virtual platform, provided to non-US health care professionals from around the world. By accessing this site and materials you accept this legal notice and expressly confirm your status as a healthcare professional.
This site is not country-specific and therefore may contain information which is not applicable to your country. Therefore, before prescribing any product, always refer to local materials such as the prescribing information and/or the Summary of Product Characteristics.
This site is not intended to provide medical advice and/or treatment guidance. Novo Nordisk accepts no liability for the accuracy, completeness or use of the information, and disclaims any liability to update the information contained on this site.
Patients with diabetes should keep daily records to achieve optimal insulin self-management2
Daily insulin self-management can be overwhelming3
Patients may face frequent, complex self-reporting:4
Insulin and/or oral medication dosing
• Glucose levels
Self-reported diabetes information is often unreliable1,5-6
Glycaemic control requires the ongoing assessment of insulin dosing patterns7
If your patients aren’t recording and reporting their dosing information, glycaemic control may be out of reach.2
And even if they are, you may not have time to review handwritten diary entries.8
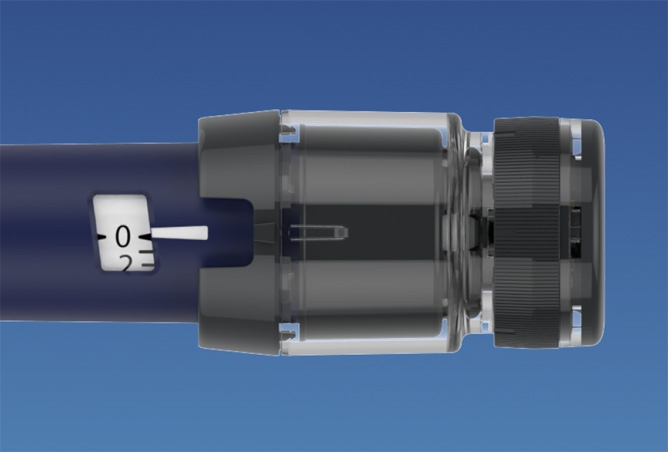
NovoPen® 6 & NovoPen Echo® Plus are smart pens that automatically record information about each injection.
Real-world insulin dosing information, can help you to personalise your patients´ diabetes management.
Reliable insulin dose recording*¹
Record
insulin dosing information
Partner
with apps and HCP portals**
Reveal
effects on glucose levels
Support
personalised treatment decisions
*Self-reported diabetes information can often be unreliable. Automatic logging may be more reliable than manual logging.¹
**When used in combination with a compatible app. NovoPen® 6 & NovoPen Echo® Plus are compatible with the following apps: mySugr®, Glooko® and FreeStyle LibreLink.
Reliable insulin dose recording1*
Potential for informed, personalised consultations based on patients´ individual injection data**
Supports treatment with Novo Nordisk insulin in cartridges
*Self-reported diabetes information can often be unreliable. Automatic logging may be more reliable than manual logging.1
**When your patients share their injection data with you via a compatible app. NovoPen® 6 & NovoPen Echo® Plus are compatible with the following apps:
mySugr®, Glooko® and FreeStyle LibreLink.
Kalergis M, Nadeau J, Pacaud D, Yared Z, Yale JF. Accuracy and reliability of reporting self-monitoring of blood glucose results in adults with type 1 and type 2 diabetes. Canadian Journal of Diabetes 2006; 30(3):241-247
Marcus A. Diabetes care - insulin delivery in a changing world. Medscape Journal of Medicine 2008; 10(5):120
Allen NA, Zagarins SE, Feinberg RG, Welch G. Treating psychological insulin resistance in type 2 diabetes. Journal of Clinical and Translational Endocrinology 2016; 7:1–6
Beverly EA, Ritholz MD, Brooks KM, Hultgren BA, Lee Y, Abrahamson MJ, Weinger K. A qualitative study of perceived responsibility and self-blame in type 2 diabetes: reflections of physicians and patients. Journal of General Internal Medicine 2012; 27(9):1180–1187
Bode BW. The accuracy and interferences in self-monitoring of blood glucose. US Endocrinology 2007; 0(2):46–48
Russell-Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes, Obesity and Metabolism 2018; 20(3):488–496
Ryan EA, Holland J, Stroulia E, Bazelli B, Babwik SA, Li H, Senior P, Greiner R. Improved A1C levels in type 1 diabetes with smartphone app use. Canadian Journal of Diabetes 2017; 41(1):33–40
Heintzman ND. A digital ecosystem of diabetes data and technology: services, systems, and tools enabled by wearables, sensors, and apps. Journal of Diabetes Science and Technology 2016; 10(1):35–41